Balanced Equation For The Titration . Ask your students to write a balanced equation of the equation before attempting any calculations. Write the balanced chemical equation for the reaction. A titration can be performed with. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. N (acid):n (base) use the stoichiometric (mole). Titration between nitric acid and sodium carbonate is represented by the equation. The example below demonstrates the. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Writing a balanced chemical equation.
from mungfali.com
A titration can be performed with. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. The example below demonstrates the. N (acid):n (base) use the stoichiometric (mole). Ask your students to write a balanced equation of the equation before attempting any calculations. Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration between nitric acid and sodium carbonate is represented by the equation. Write the balanced chemical equation for the reaction. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte).
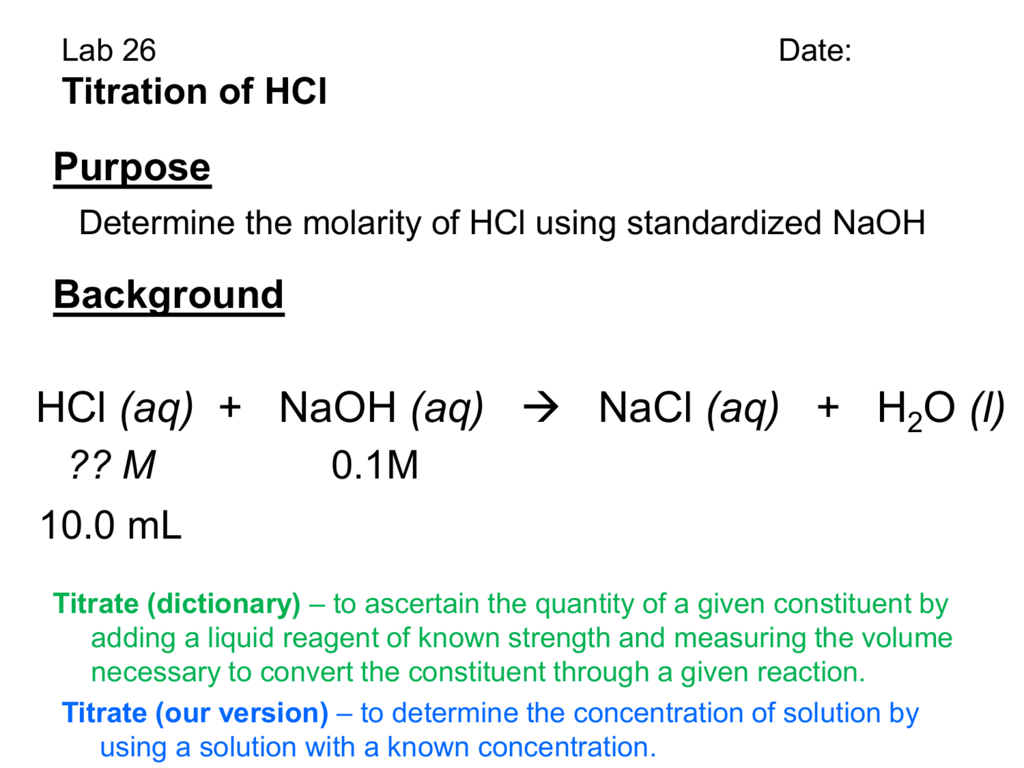
HCl NaOH Titration
Balanced Equation For The Titration Ask your students to write a balanced equation of the equation before attempting any calculations. The example below demonstrates the. N (acid):n (base) use the stoichiometric (mole). Titration between nitric acid and sodium carbonate is represented by the equation. Write the balanced chemical equation for the reaction. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A titration can be performed with. Writing a balanced chemical equation. Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Ask your students to write a balanced equation of the equation before attempting any calculations.
From www.numerade.com
SOLVED Write a complete balanced equation for the titration of Quinic Balanced Equation For The Titration N (acid):n (base) use the stoichiometric (mole). Writing a balanced chemical equation. Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: Write the balanced chemical equation for the reaction. Titration between nitric acid and sodium carbonate is represented by the equation. Ask your students to write a balanced equation of the equation before attempting. Balanced Equation For The Titration.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Balanced Equation For The Titration Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. N (acid):n (base) use the stoichiometric (mole). Write the balanced chemical equation for the reaction. A titration can be performed with. Titration between nitric acid. Balanced Equation For The Titration.
From mungfali.com
HCl NaOH Titration Balanced Equation For The Titration In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Write the balanced chemical equation for the reaction. Writing a balanced chemical equation. Titration between nitric acid and sodium carbonate is represented by the equation. Performing chemical reactions quantitatively to determine the exact amount of a reagent is. Balanced Equation For The Titration.
From mungfali.com
Acid Base Titration Calculation Balanced Equation For The Titration Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. N (acid):n (base) use the stoichiometric (mole). Titration between nitric acid and sodium carbonate is represented by the equation. Writing a balanced chemical equation. Write the balanced chemical equation. Balanced Equation For The Titration.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Balanced Equation For The Titration A titration can be performed with. Write the balanced chemical equation for the reaction. Titration between nitric acid and sodium carbonate is represented by the equation. Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. N (acid):n (base). Balanced Equation For The Titration.
From www.chegg.com
Solved Balanced Chemical Equation for Titration Reaction Balanced Equation For The Titration Titration between nitric acid and sodium carbonate is represented by the equation. N (acid):n (base) use the stoichiometric (mole). Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. A titration can be performed with. In a titration, a. Balanced Equation For The Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Balanced Equation For The Titration In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Writing a balanced chemical equation. Titration between nitric acid and sodium carbonate is represented by the equation. Performing. Balanced Equation For The Titration.
From fity.club
Titration Formula Balanced Equation For The Titration N (acid):n (base) use the stoichiometric (mole). Ask your students to write a balanced equation of the equation before attempting any calculations. Writing a balanced chemical equation. A titration can be performed with. Write the balanced chemical equation for the reaction. Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: In a titration, a. Balanced Equation For The Titration.
From www.numerade.com
SOLVED HCI NaOH NaCl H2O Balance the equation for the reaction that Balanced Equation For The Titration Ask your students to write a balanced equation of the equation before attempting any calculations. A titration can be performed with. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The example below demonstrates the. Writing a balanced chemical equation. Titration between nitric acid and sodium carbonate is represented. Balanced Equation For The Titration.
From www.reddit.com
Don't I need to know the other chemical formula to write the balanced Balanced Equation For The Titration The example below demonstrates the. A titration can be performed with. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). N (acid):n (base) use the stoichiometric (mole). Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: Write the balanced chemical equation. Balanced Equation For The Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Balanced Equation For The Titration Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Writing a balanced chemical equation. The example below demonstrates the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Ask your students to write a balanced equation of the equation before attempting any. Balanced Equation For The Titration.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Balanced Equation For The Titration The example below demonstrates the. N (acid):n (base) use the stoichiometric (mole). Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Use the balanced chemical equation to determine the stoichiometric (mole) ratio of. Balanced Equation For The Titration.
From mungfali.com
Acid Base Titration Method Balanced Equation For The Titration The example below demonstrates the. N (acid):n (base) use the stoichiometric (mole). Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: Ask your students to write a balanced equation of the equation before attempting any calculations. Titration between nitric acid and sodium carbonate is represented by the equation. A titration can be performed with.. Balanced Equation For The Titration.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Balanced Equation For The Titration Ask your students to write a balanced equation of the equation before attempting any calculations. Titration between nitric acid and sodium carbonate is represented by the equation. A titration can be performed with. Writing a balanced chemical equation. Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: The above equation works only for neutralizations. Balanced Equation For The Titration.
From www.numerade.com
SOLVED Given this balanced chemical equation and the following Balanced Equation For The Titration Ask your students to write a balanced equation of the equation before attempting any calculations. Titration between nitric acid and sodium carbonate is represented by the equation. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: Write the. Balanced Equation For The Titration.
From www.numerade.com
SOLVED Write the equation for the titration of Ca2+ with EDTA. Balanced Equation For The Titration Write the balanced chemical equation for the reaction. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Ask your students to write a balanced equation of the equation before attempting any calculations. N (acid):n (base) use the stoichiometric (mole). Titration between nitric acid and sodium carbonate is represented by. Balanced Equation For The Titration.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Balanced Equation For The Titration Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Ask your students to write a balanced equation of. Balanced Equation For The Titration.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Balanced Equation For The Titration Use the balanced chemical equation to determine the stoichiometric (mole) ratio of acid to base: A titration can be performed with. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The example below demonstrates the. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called. Balanced Equation For The Titration.